Simultaneous mapping of molecular proximity and comobility reveals agonist-enhanced dimerization and DNA binding of nuclear receptors
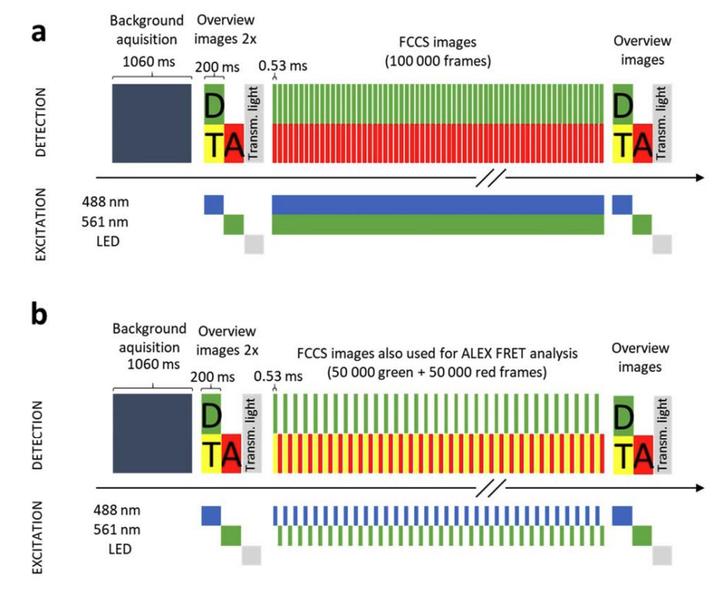 The continuous vs alternating laser excitation (ALEX) concept to co-map molecular proximity AND co-mobility in the same live cell.
The continuous vs alternating laser excitation (ALEX) concept to co-map molecular proximity AND co-mobility in the same live cell.
Abstract
Single Plane Illumination Microscopy (SPIM) revolutionized time lapse imaging of live cells and organisms due to its high speed and reduced photodamage. Quantitative mapping of molecular (co-)mobility by fluorescence (cross-)correlation spectroscopy (F(C )CS) in a Single Plane Illumination Microscope (SPIM) has been introduced to reveal molecular diffusion and binding. A complementary aspect of interactions is proximity, which can be studied by Förster resonance energy transfer (FRET). Here, we extend SPIM-FCCS by alternating laser excitation, which reduces false positive cross-correlation, and facilitates co-mapping of FRET. Thus, different aspects of interacting systems can be studied simultaneously, and molecular subpopulations can be discriminated by multiparameter analysis. After demonstrating the benefits of the method on the AP-1 transcription factor, the dimerization and DNA binding behavior of retinoic acid receptor (RAR) and retinoid X receptor (RXR) is revealed and an extension of the molecular switch model of nuclear receptor action is proposed. Our data imply that RAR agonist enhances RAR-RXR heterodimerization, and chromatin binding/dimerization are positively correlated. We also propose a ligand induced conformational change bringing the N-termini of RAR and RXR closer together. RXR agonist increased homodimerization of RXR suggesting that RXR may act as an autonomous transcription factor.