Agonist-controlled competition of RAR and VDR nuclear receptors for heterodimerization with RXR is manifested in their DNA-binding
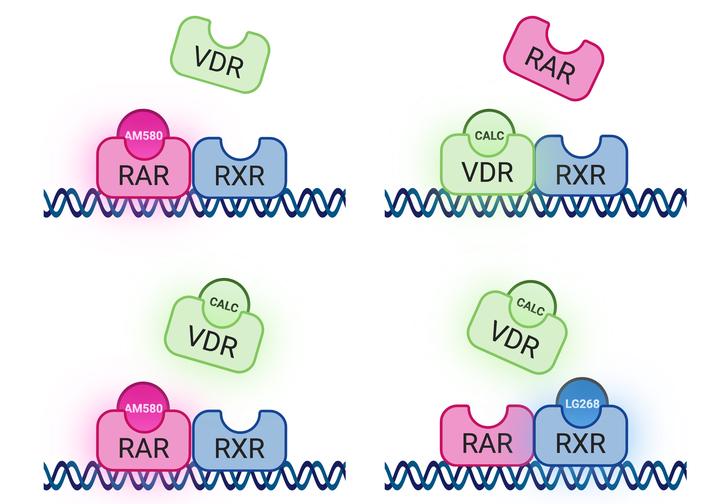 Graphical representation of the ligand-directed competition for DNA-binding between RAR and VDR. In the presence of calcitriol (top right), VDR dominates DNA-binding whereas in the presence of AM580 (top left) or both ligands (bottom left), RAR does so. The presence of RXR-specific LG268 agonist (bottom right) favors the binding of RAR.
Graphical representation of the ligand-directed competition for DNA-binding between RAR and VDR. In the presence of calcitriol (top right), VDR dominates DNA-binding whereas in the presence of AM580 (top left) or both ligands (bottom left), RAR does so. The presence of RXR-specific LG268 agonist (bottom right) favors the binding of RAR.
Abstract
We found previously that nuclear receptors (NRs) compete for heterodimerization with their common partner, retinoid X receptor (RXR), in a ligand-dependent manner. To investigate potential competition in their DNA-binding, we monitored the mobility of retinoic acid receptor (RAR) and vitamin D receptor (VDR) in live cells by fluorescence correlation spectroscopy. First, specific agonist treatment and RXR co-expression additively increased RAR DNA-binding, while both agonist and RXR were required for increased VDR DNA-binding, indicating weaker DNA-binding of the VDR/RXR dimer. Second, co-expression of RAR, VDR, and RXR resulted in competition for DNA-binding. Without ligand, VDR reduced the DNA-bound fraction of RAR and vice versa, i.e., a fraction of RXR molecules was occupied by the competing partner. The DNA-bound fraction of either RAR or VDR was enhanced by its own and diminished by the competing NR’s agonist. When treated with both ligands, the DNA-bound fraction of RAR increased as much as due to its own agonist, whereas that of VDR increased less. RXR agonist also increased DNA-binding of RAR at the expense of VDR. In summary, competition between RAR and VDR for RXR is also manifested in their DNA-binding in an agonist-dependent manner: RAR dominates over VDR in the absence of agonist or with both agonists present. Thus, side-effects of NR-ligand-based (retinoids, thiazolidinediones) therapies may be ameliorated by other NR ligands and be at least partly explained by reduced DNA-binding due to competition. Our results also complement the model of NR action by involving competition both for RXR and for DNA-sites.