Sensitivity to Vg9Vδ2TCR T cells is imprinted after single mutations during early oncogenesis
Abstract
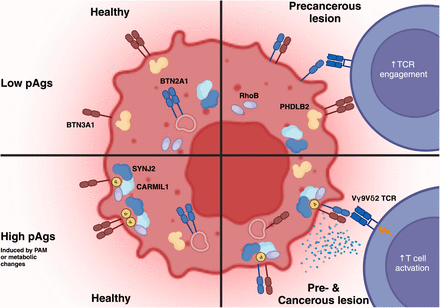
Vγ9Vδ2T cells have the unique ability to recognize a broad range of malignant transformed cells. The tumor targeting event involving BTN2A1 and BTN3A1 dimers on the tumor cell surface is critical, leading to full activation of the TCR. Although the molecular mechanisms governing TCR engagement and T cell activation are well-characterized, the role of Vγ9Vδ2 T cells in cancer immune surveillance remains to be fully elucidated, particularly the mechanisms that enable these cells to discriminate between healthy and malignant cells at an early stage of malignant transformation. We employed two independent, genetically engineered step-wise mutagenesis models of human colorectal and breast cancer that mimic the transformation steps leading to tumor formation. We demonstrate that various single oncogenic mutations introduced into healthy organoids or cells, are sufficient to upregulate surface expressed BTN2A1 and enable Vγ9Vδ2 TCR binding to tumor cells. However, full activation of T cells through a Vγ9Vδ2TCR required additional subsequent phosphorylation of juxtamembrane (JTM) amino acids of BTN3A1, leading to the activating heterodimerization of BTN2A1 and 3A1. Using a protein interactome mapping pipeline, we identified PHLDB2, SYNJ2 and CARMIL1 as key players in controlling these delicate dual surface dynamics of BTN2A1 and 3A1 during early transformation. This mode of action allowed Vγ9Vδ2TCR T cells to control tumors in vitro and in vivo, emphasizing the crucial role of these molecules from early mutagenesis, to advanced cancer stages, and highlighting the therapeutic potential of a Vγ9Vδ2TCR.